
Status (23 September): Library was submitted to Psomagen on 21 September and received on 22 September. Electropherogram QC returned 23 September. There is evidence of some high bp fragments. They are proceeding with sequencing (9-28-2020) |
183bp is coligos. 322 is Synthgene. 458 is the sample products, with a skew towards 16S over ITS.
Sequencing Report from Psomagen:
Sample ID | Total Read Bases(bp) | Total Reads | GC(%) | AT(%) | Q20(%) | Q30(%) |
NovaSeq3 | 3.82E+11 | 1.52E+09 | 63.62 | 36.38 | 90.87 | 82.85 |
Create MasterMix for a third of the reactions at a time:
ul/rxn | Reagent | # of rxns | ul needed |
|---|---|---|---|
3 | 5X Kapa HiFi Buffer | 1650 | 4950 |
0.45 | 10M dNTPs | 1650 | 743 |
0.3 | Kapa HiFi HotStart DNA Pol | 1650 | 495 |
3.25 | HPLC H2O | 1650 | 5362 |
7 | Total Volume | 1650 | 11550 |
Add 7 ul to each well of 16 hard shell, full skirt plates, Seal with tape seals, rub with kimwipe across all wells twice, and then around the edge, store in refrigerator until needed labeled “PCR1”. (If doing manually, one needs 2 ul of template and 6 ul of the primers).
Spin down 4 “PCR1” plates. Vortex and spin 1 “_Norm” plate. Vortex and spin to 16S MID plates and 2 ITS MID plates.
Open “TwoLociDuplicatePCR_Prep_BR” in the Nimbus Method Editor
Press the stop light icon to open Hamilton Run Control
Press the green triangle
Load 3 of the MID plates, the 4 PCR1 plates, and the “_Norm” plate (template) according to the prompts and 5 of the 50 ul conductive, sterile, filtered tips.
When prompted, swap the “_Norm” with the 4th MID plate.
Spin down resulting “PCR1”s and then add to thermocyclers running “35GSAF1”
Temp C | Cycles | Time |
|---|---|---|
95* | 1X | 3:00 |
98 | 15X | 0:30 |
62 | 15X | 0:30 |
72 | 15X | 0:30 |
72 | 1X | 5:00 |
4 | 1X | 0:00 |
Store at 4C when cycler reaches below 40C unless immediately proceeding to next step
This was done on the Nimbus platform using “AxyPrep MagBead PCR1 No MM”
and manually. Probably 3/4 of the plates were cleaned manually.
Manual protocol
Equilibrate Beads to room Temperature
Add 24 ul of MagBeads to each well; Pipette mix up and down 10 times.
Incubate at RT for 5 minutes
Secure plate on magnet plate; incubate at RT for 5 minutes (until wells are clear)
Remove 65 ul from each well; keep tips to left or right depending on the column to avoid bead pellet.
Add 100 ul Fresh 80% EtOH to each well. Incubate 30 seconds. Remove 100 ul from each well
Add 100 ul Fresh 80% EtOH to each well. Incubate 30 seconds. Remove 100 ul from each well
Reaspirate from each well to assure maximum EtOH removal
Allow plate to air dry for 7 minutes.
Remove sample plate from magnet plate.
Add 30 ul H2O; pipette mix 10+ times. Incubate 2 minutes at RT.
Place sample plate back on magnet for 5 minutes or until all wells are cleared.
Transfer 30 ul to labeled transparent plate (Plate1 PCR1 MIDPlate1 MIDPlate2)
Transfer 10 ul from transparent plate to “PCR2” plate with Mastermix already added.
Label Plate1 PCR2 MIDPlate1 MIDPlate2
Seal “PCR2”s with bubble strips and run on thermocycler 35GSAF2 program
Create MasterMix2 for 1/3 of the reactions:
ul/rxn | Reagent | # of rxns | ul needed |
|---|---|---|---|
3 | 5X Phusion HF Buffer | 810 | 2430 |
0.45 | 10M dNTPs | 810 | 364.5 |
0.3 | Kapa HiFi HotStart DNA Pol | 810 | 243 |
0.5 ul | 5 uM F and R FlowCell Primers | 810 | 405 |
0.75 | HPLC H2O | 810 | 607.5 |
5 | Total Volume | 810 | 4050 |
Add 5 ul master mix to all wells of 4 hard shell full skirted plates (the cheaper soft, skirted plates don’t seal very well regardless of caps used). I am using the new green plates Gregg got. Seal with tape seals and store in refrigerator if not using immediately. Add 10ul of template from PCR1.
Temp C | Cycles | Time |
|---|---|---|
95* | 1X | 3:00 |
98 | 20X | 0:30 |
55* | 20X | 0:30 |
72 | 20X | 0:30 |
72 | 1X | 5:00 |
4 | 1X | 0:00 |
Equilibrate Beads to room Temperature
Add 15 ul H2O to each sample
Add 24 ul (0.8 x 60 ul) of MagBeads to each well; Pipette mix up and down 10 times
Incubate at RT for 5 minutes
Secure plate on magnet plate; incubate at RT for 5 minutes (until wells are clear)
Remove 54 ul from each well; keep tips to left or right depending on the column to avoid bead pellet.
Add 100 ul Fresh 80% EtOH to each well. Incubate 30 seconds. Remove 100 ul from each well.
Add 100 ul Fresh 80% EtOH to each well. Incubate 30 seconds. Remove 100 ul from each well.
Allow plate to air dry for 7 minutes.
Remove sample plate from magnet plate.
Add 40 ul TE per GSAF protocol; pipette mix 10 times
Incubate at RT for 2 minutes
Place sample plate back on magnet for 5 minutes or until all wells are cleared.
Transfer 40 ul to a clean transparent PCR plate labeled “Plate1 PCR2 MIDPlate1 MIDPlate2
Benchsmart 96 used to transfer 2 ul from each well of 24 plates to a
Bioanalyzer:
Ran Yoon Yoon Plate by itself and the rest of the pool by itself on the bioanalyzer
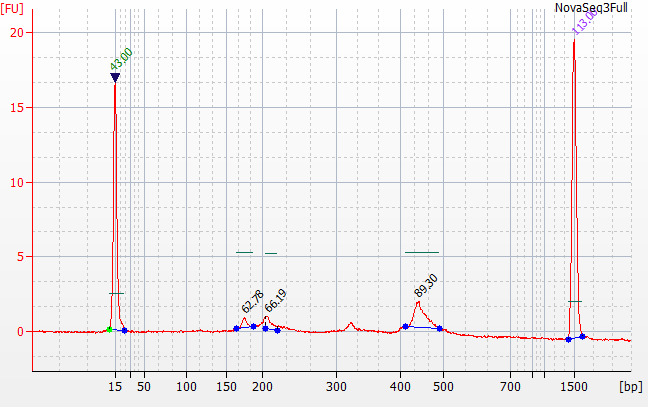
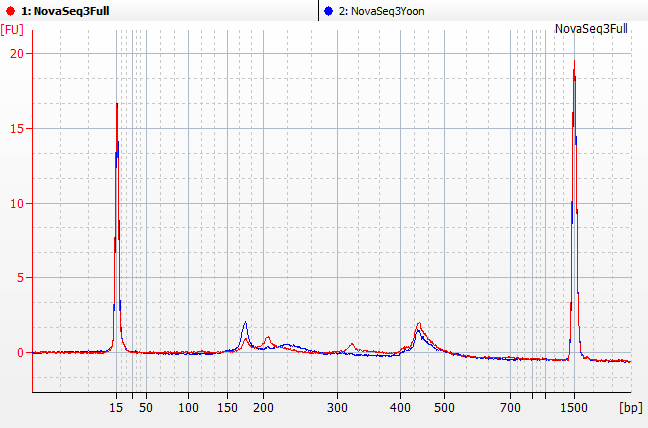
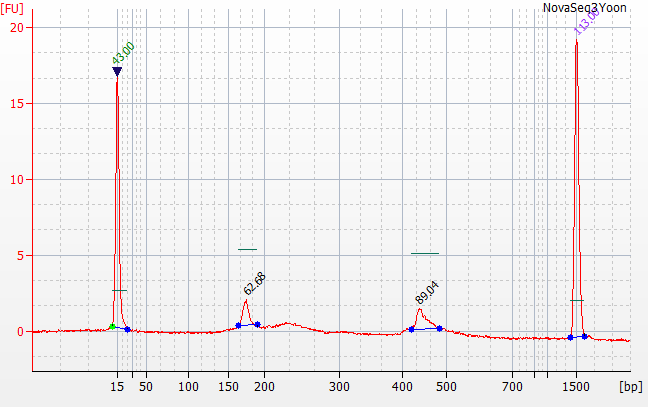
We initially tried qPCR on the full strength pool and a 1:1000 dilution.
ul/rxn | Reagent | # of rxns | ul needed |
|---|---|---|---|
10 ul | KAPA SYBR FAST qPCR MM (2X) | 24 | 300 |
2 ul | Primer Premix (10X) | 24 | 60 |
4 ul | Ultra Pure Water | 24 | 120 |
16 ul | Total Volume | 24 | 480 |
Add 4 ul of 1:1000 templates and standards
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
A | NTC | NTC | NTC | NovaSeqYoon | ||||||||
B | 0.0002 pM Std | 0.0002 pM Std | 0.0002 pM Std | NovaSeqYoon | ||||||||
C | 0.002 pM Std | 0.002 pM Std | 0.002 pM Std | NovaSeqYoon | ||||||||
D | 0.02 pM Std | 0.02 pM Std | 0.02 pM Std | |||||||||
E | 0.2 pM Std | 0.2 pM Std | 0.2 pM Std | |||||||||
F | 2 pM Std | 2 pM Std | 2 pM Std | |||||||||
G | 20 pM Std | 20 pM Std | 20 pM Std | |||||||||
H | NovaSeq3 | NovaSeq3 | NovaSeq3 |
Both pools were 20-25 nMolar with Yoon being closer to 20 and the Full being closer to 25.